calcium molar mass|More : Tuguegarao Calculate the molar mass of any element or compound using the atomic masses of its constituents. Learn the definition, formula and examples of . Resultado da Event by Vestcasa on Monday, April 9 2018 with 643 people interested and 105 people going.
0 · molar mass of calcium nitrate
1 · molar mass calcium sulfate
2 · molar mass calcium bromide
3 · molar mass calcium bicarbonate
4 · how much does calcium weigh
5 · calculating molar mass
6 · More
7 · 45 atoms of calcium weigh
8 · 2.00 mol of calcium atoms
WEB19 de mar. de 2020 · Carol vem sofrendo constantes críticas e ataques por sua forma física imperfeita. Com a segunda gravidez, obviamente, não pode evitar o ganho de peso, mas ela afirma não se importar com os insultos. Ela assume, no entanto, que possui próteses de silicone nos seios. Confira a seguir a melhor seleção de fotos da Carol Dantas nua:
calcium molar mass*******Learn how to find the molar mass of a substance by summing the molar masses of its component atoms, and how to use it to convert between mass and number of moles. See examples, video, and comments on this chemistry topic.Calcium molecular weight. Molar mass of Ca = 40.078 g/mol. Convert grams Calcium to moles. or. moles Calcium to grams. Percent composition by element. Element: Calcium .
Calculate the molar mass of any element or compound using the atomic masses of its constituents. Learn the definition, formula and examples of .
calcium molar massCalcium is a very ductile silvery metal (sometimes described as pale yellow) whose properties are very similar to the heavier elements in its group, strontium, barium, and radium. A calcium atom has twenty electrons, with electron configuration [Ar]4s . Like the other elements placed in group 2 of the periodic table, calcium has two valence electrons in the outermost s-orbital, which are v.Element Calcium (Ca), Group 2, Atomic Number 20, s-block, Mass 40.078. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main .
So, the molar mass of calcium would be 40 g/mol. Answer link. 40 \ "g/mol". On the periodic table, it shows us that calcium has a mass of around 40 \ "amu". We .calcium molar mass MoreUse the molar mass of H 2 O as a conversion factor from mass to moles. The molar mass of water is (1.0079 + 1.0079 + 15.999) = 18.015 g/mol. However, because we want to .
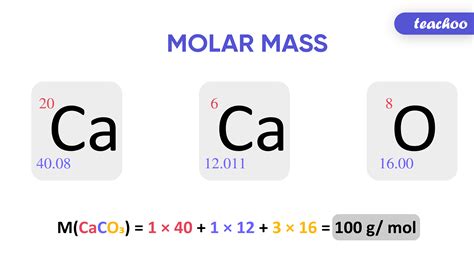
Total Mass. 40.078. The molar mass of Ca (Calcium) is: 40.078 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the .Learn how to calculate the molar mass of calcium and other substances using the formula Molar mass = Atomic mass of element × number of atoms given in subscript. Find . Similarly, the formula mass of calcium phosphate [Ca 3 (PO 4) 2] is 310.177 amu, so its molar mass is 310.177 g/mol. This is the mass of calcium phosphate that contains 6.022 × 10 23 formula units. The mole is the basis of quantitative chemistry. So, the molar mass of calcium would be 40 g/mol. Answer link. 40 \ "g/mol". On the periodic table, it shows us that calcium has a mass of around 40 \ "amu". We now need to convert from "amu" to "g/mol". We know that the mass of one mole of carbon-12 atoms is 12 \ "g", or, 1 \ "mol"*m_ (""^12C)=12 \ "g", because of the definition of the mole.
Total Mass. 40.078. The molar mass of Ca (Calcium) is: 40.078 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be atoms, molecules, or formula units of ionic compounds. . Calculate the mass of 3.00 moles of calcium chloride (CaCl 2). Figure \(\PageIndex{1}\): Calcium chloride is used as a .The molar mass of any compound is the mass in grams of one mole of that compound. One mole of carbon dioxide molecules has a mass of 44.01g 44.01 g, while one mole of sodium sulfide formula units has a mass of 78.04g 78.04 g. The molar masses are 44.01g/mol 44.01 g/mol and 78.04g/mol 78.04 g/mol respectively. In both cases, that is .Q. Calculate the molar mass of Calcium Hydroxide. Q. Avagadros no. Is changed from 6.023*10^23 to 3*10^23 what is the molar mass of calcium. Q. The volume of CO2 obtained at STP by the decompostion of 10 g of calcium carbonate is: (molar mass of calcium carbonate = 100 gmol−1. View More.Finally, add together the total mass of each element to get the molar mass of Ca (NO3)2: 40.078 g/mol + 28.0134 g/mol + 95.9964 g/mol = 164.0878 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in Ca (NO3)2, divide each total from step 3 by the total molar mass found in step 4:
The molar mass for this compound is computed to be 176.124 g/mol. The given number of moles is a very small fraction of a mole (~10 −4 or one-ten thousandth); therefore, we would expect the corresponding mass to be about one-ten thousandth of the molar mass (~0.02 g). Performing the calculation, we get:
Lastly, add the atomic masses of each element in the compound to get the molar mass of the compound. In this case, we add the atomic masses of calcium and chlorine to get the molar mass of calcium chloride: Molar mass of CaCl_2 = 40.08\text{ g/mol} + 70.90\text{ g/mol} = 110.98\text{ g/mol} The mass of a mole of substance is called the molar mass of that substance. The molar mass is used to convert grams of a substance to moles and is used often in chemistry. . How many grams are 10.78 moles of Calcium (\(\ce{Ca}\))? Solution. Multiply moles of Ca by the conversion factor (molar mass of calcium) 40.08 g Ca/ 1 .
Calcium (Ca2+) plays a pivotal role in the physiology and biochemistry of organisms and the cell. It plays an important role in signal transduction pathways, where it acts as a second messenger, in neurotransmitter release from neurons, contraction of all muscle cell types, and fertilization.The first step to finding the molar mass of Calcium Acetate is to count the number of each atom present in a single molecule using the chemical formula, Ca(C2H3O2)2: Element Number of Atoms; Ca (Calcium) 1: C (Carbon) 4: H (Hydrogen) 6: O (Oxygen) 4: 2. Find Atomic Mass of Each Element.Finally, add together the total mass of each element to get the molar mass of CaCl2: 40.078 g/mol + 70.906 g/mol = 110.984 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in CaCl2, divide each total from step 3 by the total molar mass found in step 4: Mole Fraction. Percent.Finally, add together the total mass of each element to get the molar mass of CaF2: 40.078 g/mol + 37.9968064 g/mol = 78.0748064 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in CaF2, divide each total from step 3 by the total molar mass found in step 4: Mole Fraction. Percent.

Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium.Calculate the molar mass of Calcium in grams per mole or search for a chemical formula or substance.
Ca (Calcium) molar mass. Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of Ca (Calcium) is 40.0780 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between Ca weight and moles. Compound.Element Calcium (Ca), Group 2, Atomic Number 20, s-block, Mass 40.078. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.The molar mass of a substance is the mass in grams of 1 mole of the substance. As shown in this video, we can obtain a substance's molar mass by summing the molar masses of its component atoms. We can then use the calculated molar mass to convert between mass and number of moles of the substance.
Our molar mass calculator comes to the rescue if you need to quickly check the weight of 1 mole of any element or chemical compound and you are unable to use the periodic table. Simply select one by one the elements from the list and give the number of atoms in their molecular formula to get the molar mass in a flash.MoreThe molar mass (in grams) is the mass of one mole of a compound or element. The molar mass is a useful conversion factor, which can be used to convert from grams to moles or from moles to grams. On the periodic table, it shows us that calcium has a mass of around 40 \ "amu". We now need to convert from "amu" to "g/mol". We know that the mass of one mole of carbon-12 atoms is 12 \ "g", or, 1 \ "mol"*m_(""^12C)=12 \ .
webCom o PegaJogo você baixa qualquer jogo online para seu PC. De qualquer site que quiser. Você baixa o PegaJogo e salva o jogo que quiser de .
calcium molar mass|More